Say goodbye to
regulatory & medical writing
Save time for your medical and regulatory teams!
The fastest way to generate
compliant regulatory documentation
From data extraction to final draft, Syntro transforms fragmented workflows into one smart, unified system for faster, easier submissions.
The document you work on is missing?
Designed for the complexity
of live sciences documentation
Syntro isn’t just about faster documents. It’s about transforming how pharma and CROs create, manage, and deliver compliant content.
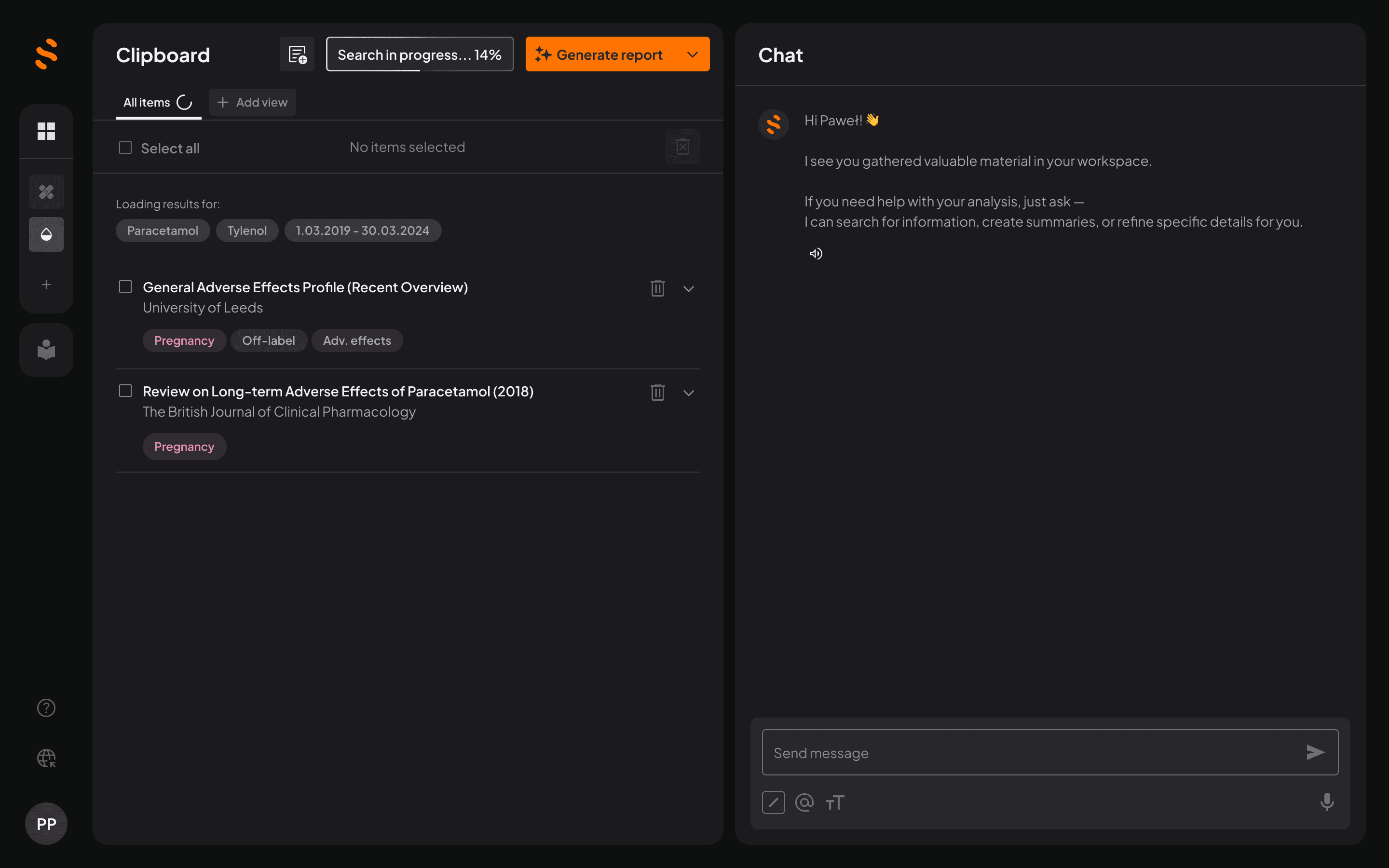
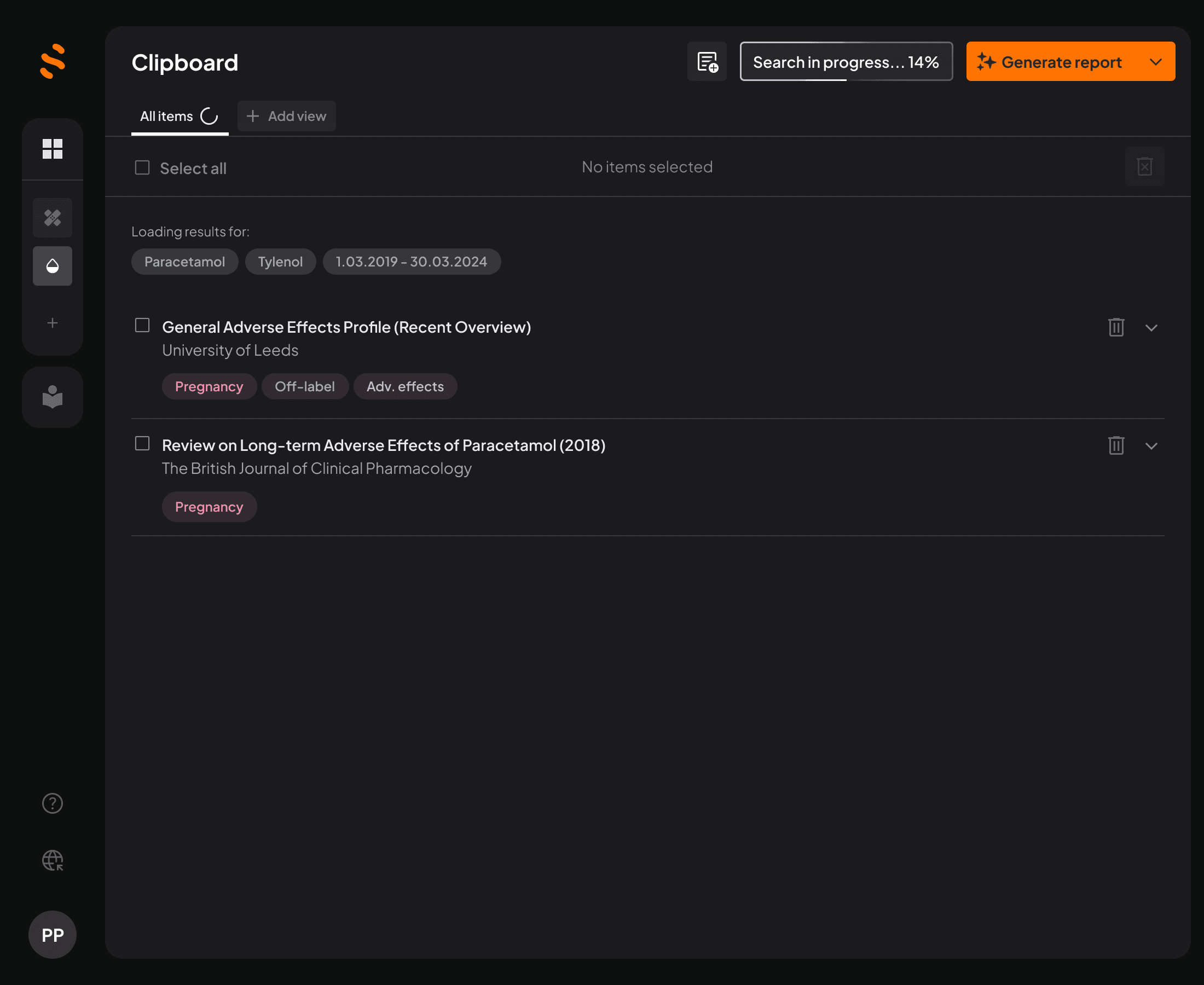
Quickly find, filter, and extract the right data from scientific publications and public sources – no more hours wasted digging.
Automated drafting
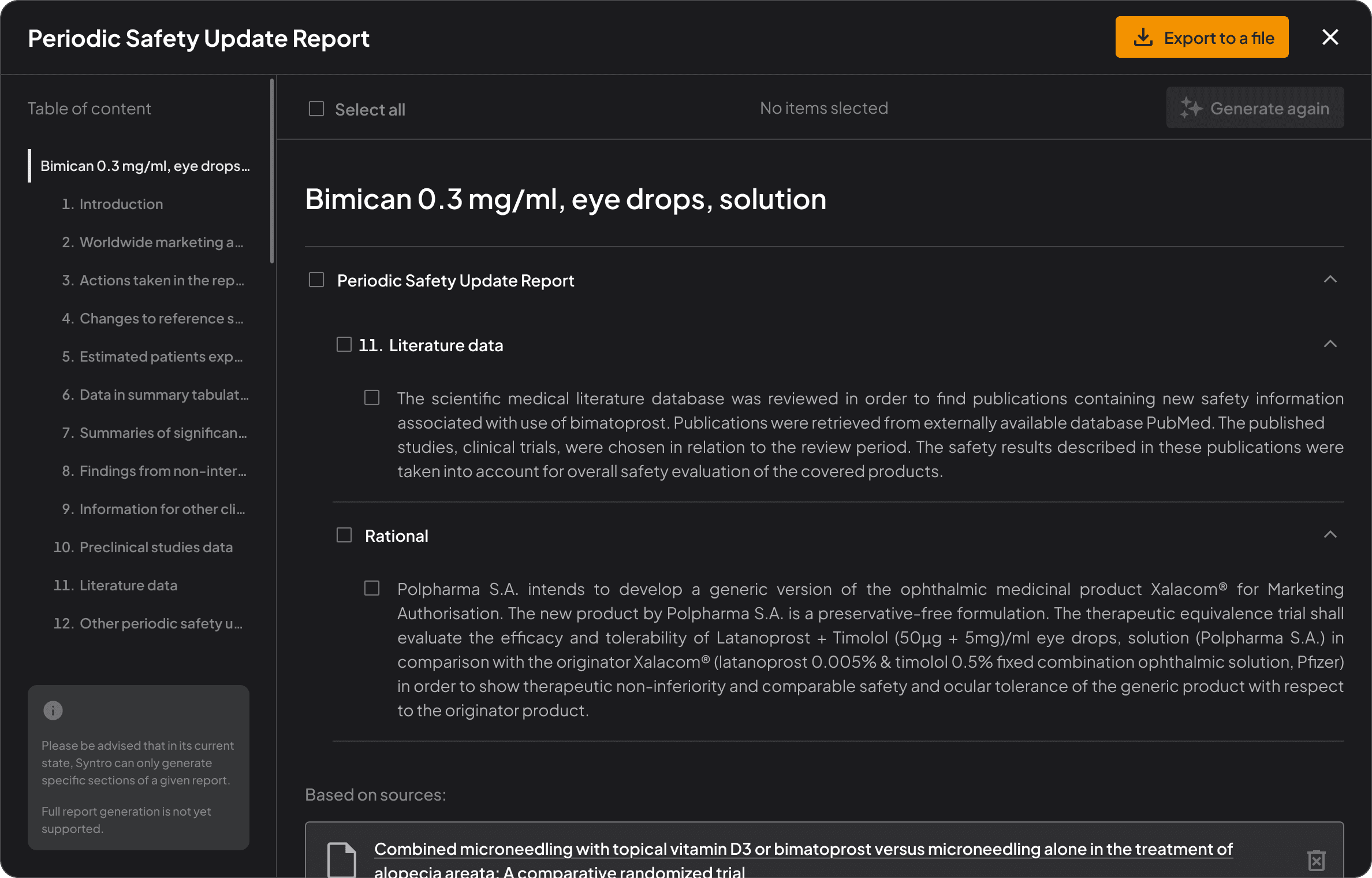
Deep research assistant
Ask any scientific question – get accurate, source-backed answers in seconds.
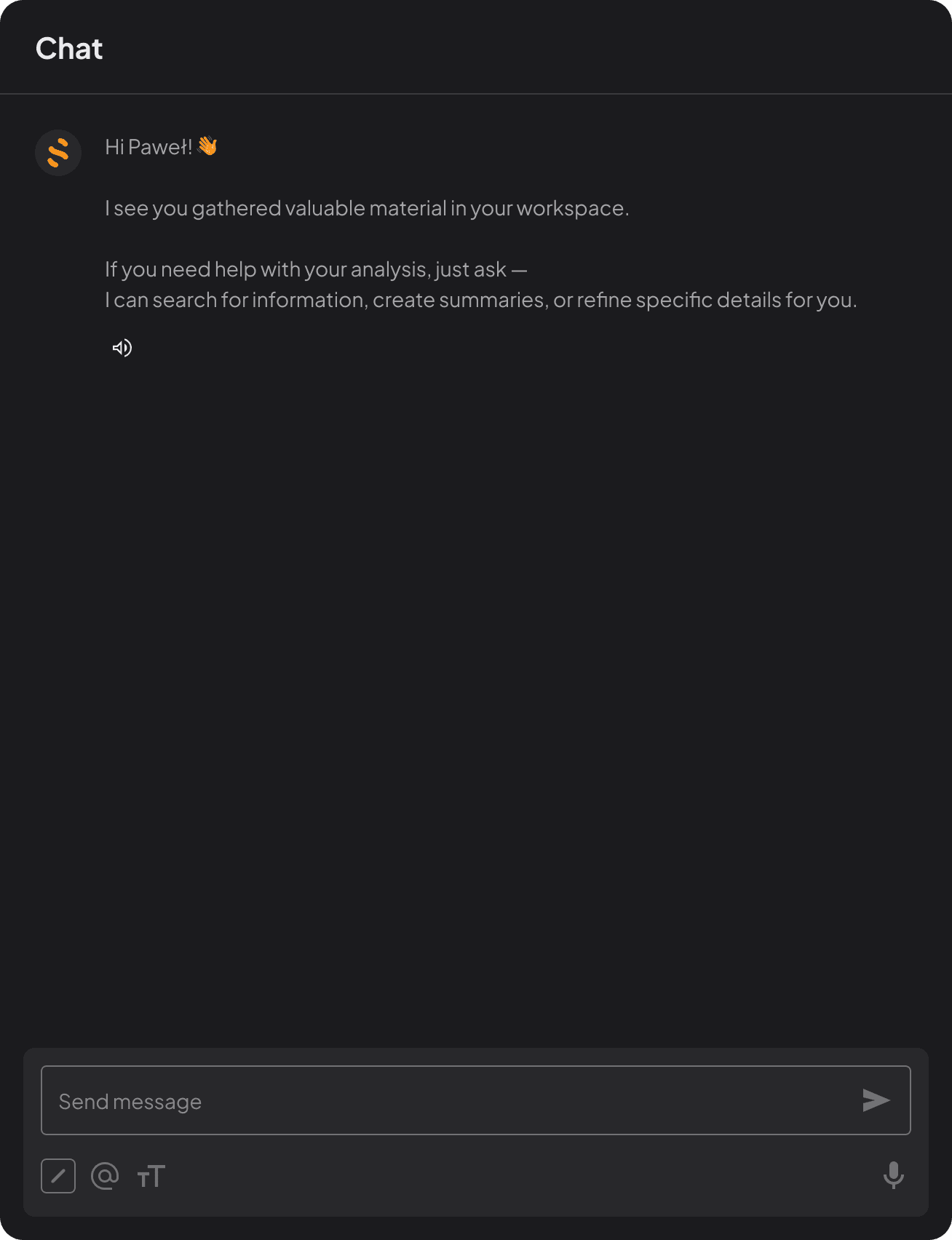
Source traceability
Track every AI-generated output back to its original source instantly.
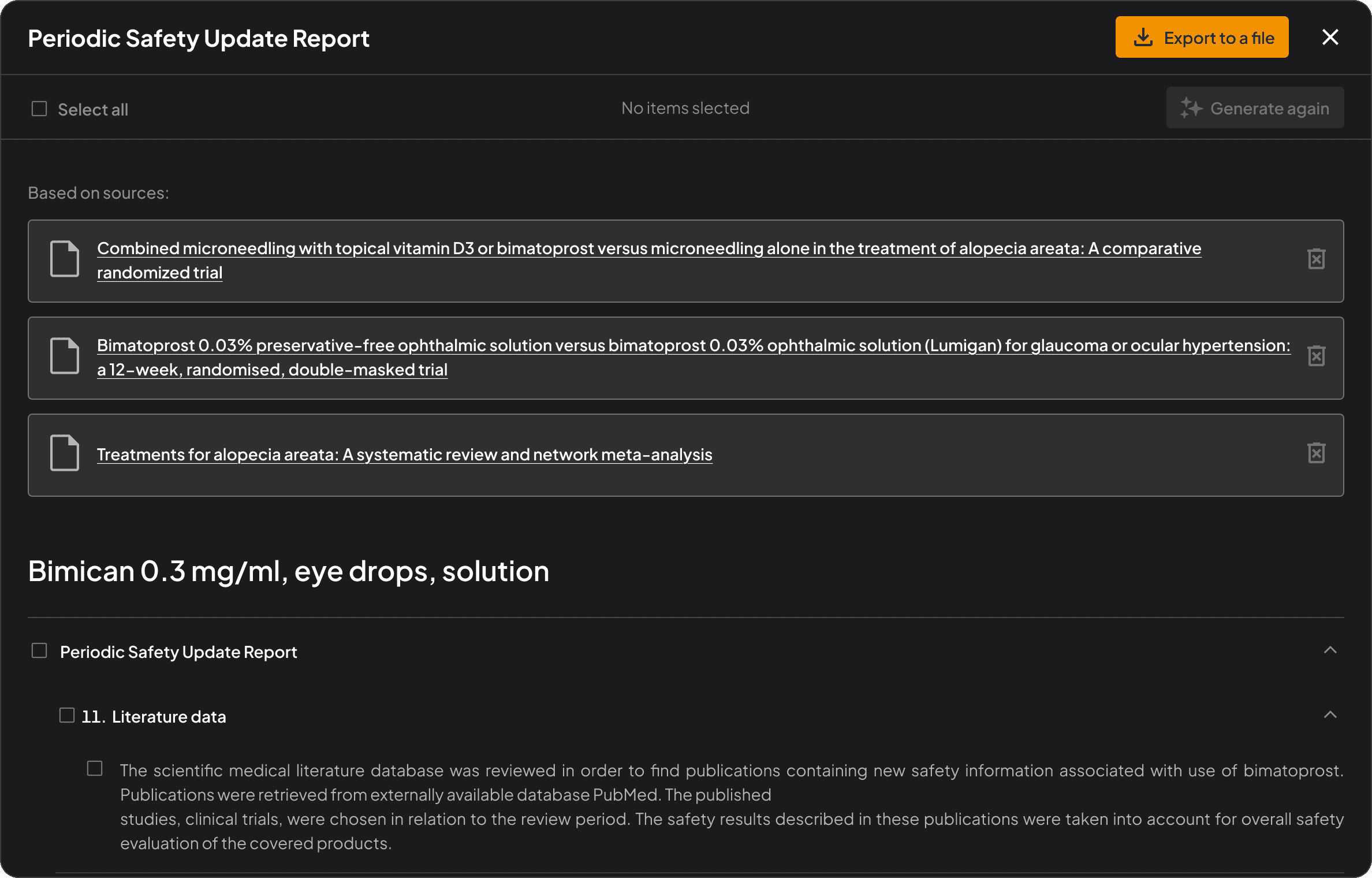
Built-in regulatory intelligence
Stay aligned with EMA, FDA, and ICH guidelines automatically, as you write.
Aligned with:
From faster submissions to lower costs and audit-ready documents – Syntro gives you measurable impact from day one.
Cut your IND, NDA, or CTA timelines by up to 5 times – without sacrificing quality or compliance.
Automate the most time-consuming tasks – literature review, data extraction, formatting – to lift the burden from your team, so they can focus on strategy, not grunt work.
Avoid hundreds of thousands spent drafting the same documents from scratch.
Trust the output – zero hallucinations means your team doesn’t waste time fact-checking or fixing misleading content.
Ensure instant compliance with EMA and FDA guidelines, so your documentation is submission‑ready from day one.
Effectiveness backed
by numbers
Hours saved for regulatory and medical writing teams
Drafts created
Accuracy
Halucinations
Learn more about Syntro
Our platform integrates seamlessly with tools: SharePoint, PubMed, and Veeva to ensure smooth data flow and minimal disruption.
Syntro is built with advanced security features to protect your data, ensuring confidentiality and preventing unauthorized access.
We uphold the highest legal standards, ensuring that your data is handled responsibly and in full compliance with international regulations.
Collaborating across disciplines
to revolutionize regulatory writing
Our mission is to change the way medical and regulatory writers handle documentation.








Potential recognized
Partnered with innovators across life sciences
Supported by leading acceleration programs